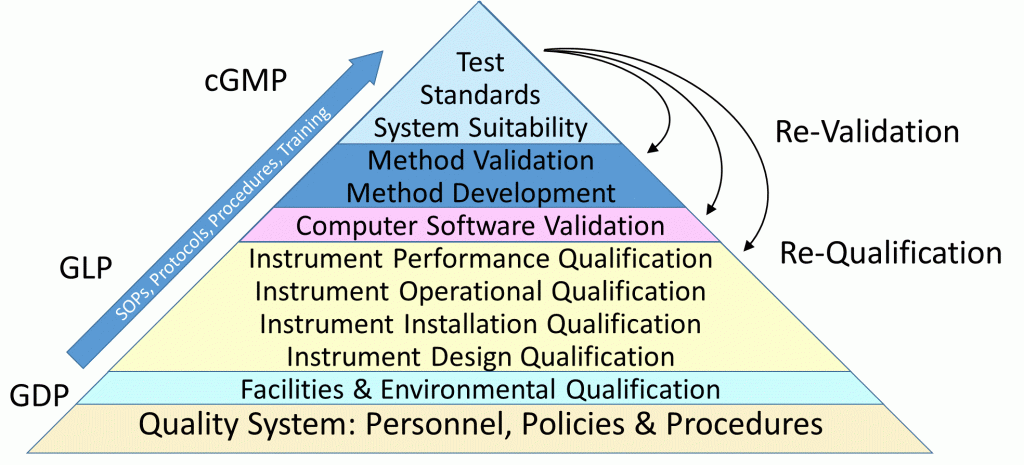
Essential to ensuring quality and reliability are suitably validated systems. This encompasses the entire pharmaceutical quality system ranging from facilities, environment, equipment, software, processing (compounding, manufacturing, packaging, labeling), product release, testing and shipping. Various degrees of validation is also applicable to the various stages of product development or use – exploratory, developmental, clinical, final product and stability.
Validations deemed suitable for their intended use incorporates RISK and the STAGE of USE in determining the specific tests and the extent of those tests to be performed to meet both business needs and guidance requirements. Also potentially impacting the depth and type of testing can be the timing of a validation – before use, concurrent, retrospective and revalidation.
With over thirty years of experience in meeting regulatory and business requirements – support is provided for all stages of qualification and validations, with extensive expertise in instrument qualification and test method validation, including computer software validation.
- Analytical Test Method Verification
- Analytical Test Method Validation
- Computer Software Validation
- Cleaning Validation
- Real-time Release Validation
- Process Validation
- Instrument Qualification
(aka Pharma-Validations Pharma-Validations.com)
