A prerequisite to a validated system is the qualification of all supporting equipment / instrumentation. This additionally includes the environment, operators / analysts and computer software. This is described by USP <1058> for risk based analytical instrument qualification. While many vendors and the instrument supplier provide qualification, it remaining the users responsibility as to establishing the appropriateness and suitability of the qualification performed, incorporating the environment, controls, standards, user training, security, and all related supporting documents.
Risk
The actual requirements vary based upon specific use and associated product safety and risks. This is based upon a prior documented scientifically justified risk assessment (e.g., FMEA). This risk assessment can also serve as the basis for the appropriateness of subsequent method validation criteria.
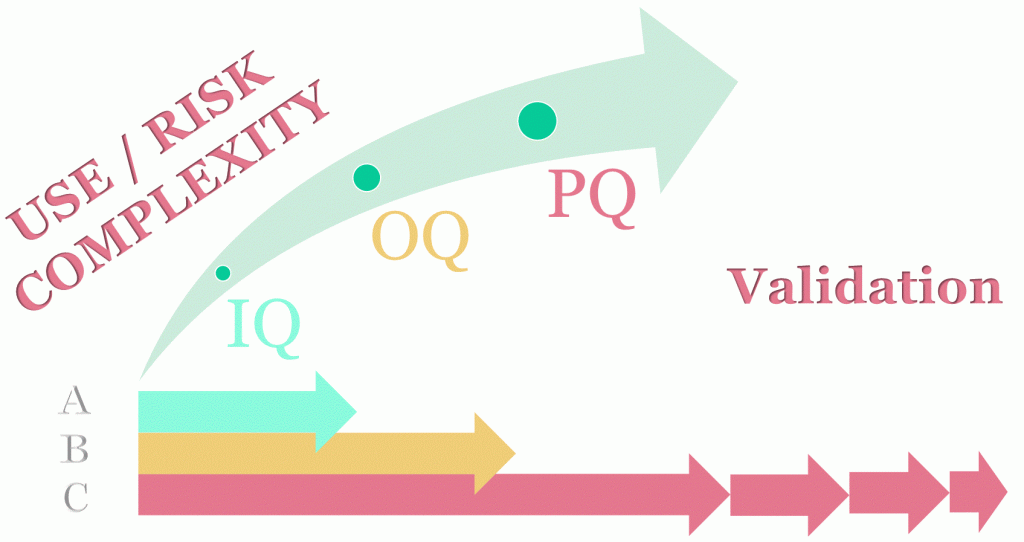
Validation / Qualification V-Model